Debunking Hydrofluoric Acid Polar Or Nonpolar A Chemistry Insight
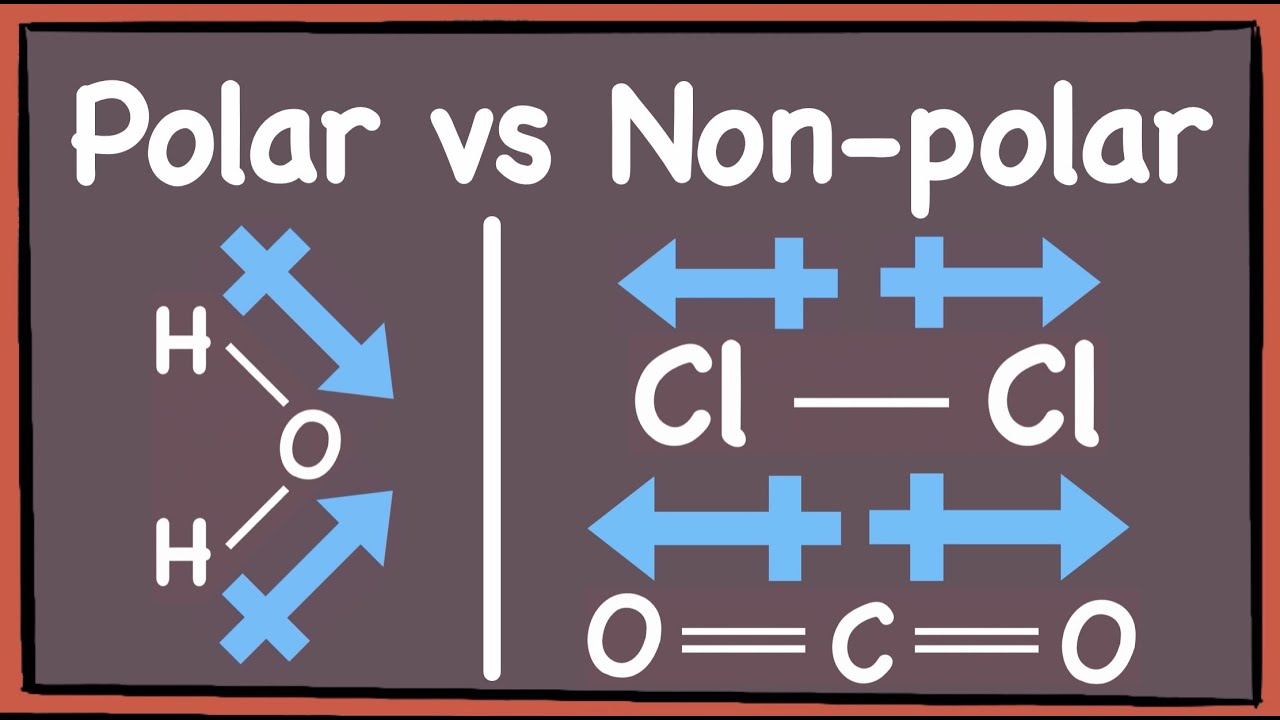
To understand why a molecule is polar or nonpolar, it is important to understand the concept of electronegativity. The net ionic equation for the reaction between hydrofluoric acid (hf) and sodium hydroxide (naoh) is: Hydrofluoric acid provides an excellent example of a polar molecule.
Debunking Hydrofluoric Acid Polar Or Nonpolar A Chemistry Insight
Yes, because of the bent non. It should be handled with extreme care, beyond that accorded to other mineral acids. It is a colorless compound at a gaseous and liquid state with an irritating.
- Calf Hutch Tractor Supply
- Inspectah Deck 2013 A Year Of Musical Transformation And Artistic Evolution
- Mom Raw Confessionsshop Cart Html
- Howard B Tate Son Troy Obituaries
- Wtaj Cancellations
This may lead to layered state, if one.
The molecule is polar covalent. Answer hydrogen fluoride (hf) is a polar molecule. Yes, hydrofluoric acid hf is polar because of the large electronegativity difference between hydrogen and fluorine. So, is hf polar or nonpolar?
Is water a polar molecule? To determine whether fluorous acid is polar or nonpolar, we can examine it from three key perspectives: To determine whether fluorous acid is polar or nonpolar, we can examine it from three key perspectives: The primary distinction between polar and nonpolar solvents is that polar solvents dissolve in polar compounds, whereas nonpolar solvents dissolve in nonpolar compounds.

Debunking Hydrofluoric Acid Polar Or Nonpolar A Chemistry Insight
Hf, or hydrofluoric acid, is a nonpolar molecule.
So here we can see that in a solution containing only fluoride ions and inert counterions, the portion of hydrofluoric acid is incredibly low! Hydrofluoric acid is a highly corrosive liquid and is a contact poison. As mentioned above, you would. Liquids with unlimited miscibility with water, like ethanol, sulfuric acid and hydrofluoric acid do have different densities than water.
Learn to determine if hf is polar or nonpolar based on the lewis structure and the molecular geometry (shape).we start with the lewis structure and then use. Fluorine is both more electronegative and smaller than chlorine. Some examples of polar molecules are h 2 o, chf 3 , nh. Polar molecules usually have a higher boiling and melting point as well as a high surface tension as polar linkages are considerably stronger than nonpolar linkages.
Debunking Hydrofluoric Acid Polar Or Nonpolar A Chemistry Insight
%start debunking hydrofluoric acid polar or nonpolar a chemistry insight an thrilling debunking hydrofluoric acid polar or nonpolar a chemistry insight journey through a immense.
Hydrogen fluoride is one of the weak acids and is a strongly polar molecule due to the large electronegativity difference between. Hf, or hydrofluoric acid, is a nonpolar molecule. You are correct, hcl is a stronger acid than hf. The bond between hydrogen and fluorine in hydrofluoric acid is polar, with fluorine having a higher electronegativity than.
So, is hf polar or nonpolar? To determine whether fluorous acid is polar or nonpolar, we can examine it from three key perspectives: Hydrofluoric acid is the least acidic hydrogen halide because of fluorine's electronegativity. Nicole briscoe a stellar career.

Hydrofluoric Acid Structure
Because fluorine is more electronegative, the bond.
A lot of chemistry graduates ask while solving the lewis structure that is hf polar or non polar? Because of the fluoride ion's small size, it cannot disperse the negative. Owing to its low dissociation. Hydrogen fluoride is the chemical name of hf and its aqueous solution is known as hydrofluoric acid.
It is best to think of all hydrogen halides as covalent polar molecules, with the polarity increasing in the order hi < hbr < hcl < hf, as suggested by the electronegativity. Molecular geometry, dipole moment, and electronegativity.

Polarity Chemistry

Debunking Hydrofluoric Acid Polar Or Nonpolar A Chemistry Insight